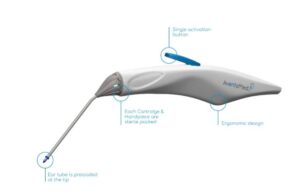
Karl Storz’s AventaMed wins FDA nod for ear tube placement device
AventaMed, a Karl Storz company, announced that it received FDA 510(k) clearance for its Solo+ ear tube placement system.

AventaMed, a Karl Storz company, announced that it received FDA 510(k) clearance for its Solo+ ear tube placement system.

Medtronic (NYSE: MDT)+ got another boost for its CGM portfolio, adding to the collaboration with Abbott announced today.
CINCINNATI, OH August 7, 2024 – Ethicon*, a Johnson & Johnson MedTech company**, announced today that the U.S. Food & Drug Administration (FDA) has approved a label update to expand the availability of the LINX™ Reflux Management System to include patients with Barrett’s esophagus (BE) experiencing gastroesophageal reflux disease (GERD) symptoms.

IRVINE, Calif., Aug. 6, 2024 /PRNewswire/ — Aspen Medical Products (Aspen), the industry leader in spine solutions for pain and mobility management, has launched the Align Orthosis. In collaboration with spine surgeon Han Jo Kim, MD of the Hospital for Special Surgery, the all-new Align Orthosis is the first prefabricated TLSO designed to help mitigate post-surgical incidence of proximal junctional kyphosis (PJK).
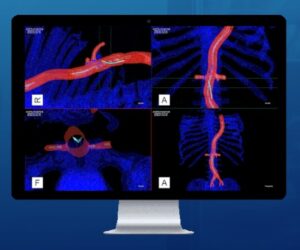
CLEVELAND, Aug. 6, 2024 /PRNewswire/ — Centerline Biomedical, Inc. (“Centerline”), an innovation leader in cardiovascular navigation and visualization systems, announced today that the IOPS Viewpoint Catheter has received US Food and Drug Administration (FDA) 510(k) clearance. The Viewpoint Catheter is the most recent addition to the company’s patented IOPS (Intra-Operative Positioning System) portfolio.
Stiffness, pain and infections in orthopedic surgery is being tackled by Flinders University researchers driving innovation in alloy materials to produce safe and superior implants compatible with human tissue.

Stryker (NYSE: SYK)+
announced today that it launched its Pangea plating system portfolio of devices.

Diality announced today that it received FDA 510(k) clearance for its Moda-flx smart, flexible hemodialysis system.
Northwestern University scientists have developed a new bioactive material that successfully regenerated high-quality cartilage in the knee joints of a large-animal model.

Pentax Medical announced today that the FDA cleared its DEC duodenoscope compatibility with the Sterrad 100NX sterilizer.