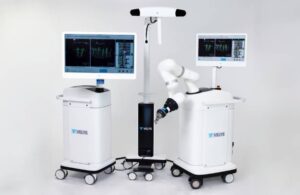
J&J’s DePuy Synthes launches Velys surgical robot for spine
Johnson & Johnson’s (NYSE: JNJ)+
DePuy Synthes announced today that it launched a dual-use robotics and standalone navigation platform that uses its Velys robot in spine surgery.

Johnson & Johnson’s (NYSE: JNJ)+
DePuy Synthes announced today that it launched a dual-use robotics and standalone navigation platform that uses its Velys robot in spine surgery.
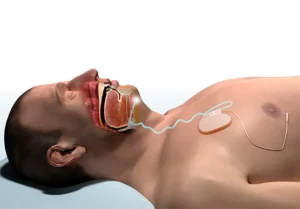
Inspire Medical Systems (NYSE: INSP)+ announced today that it received FDA approval for its Inspire V therapy system.
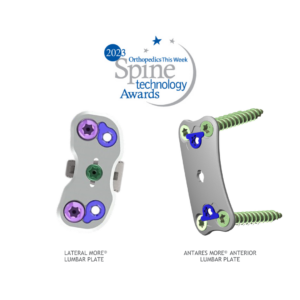
MiRus announced that it received FDA breakthrough device designation for its Europa posterior cervical system for the spine.

Perceptive today announced the completion of a fully automated dental procedure on a human using its advanced robotic system.
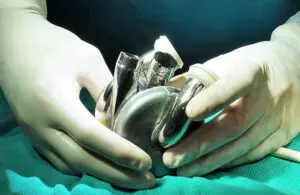
BiVacor recently announced the successful first-in-human implantation of its Total Artificial Heart (TAH) as part of an early feasibility study.
Virtual Incision today announced the completion of the first surgery in a study assessing its surgical robot for hysterectomy procedures.

Stryker (NYSE: SYK)+
announced today that it received FDA 510(k) clearance for its Q Guidance System with Spine Guidance 5 software featuring Copilot.
Siemens Healthineers‘ Varian announced that it received FDA 510(k) clearance for its IntelliBlate microwave ablation system.
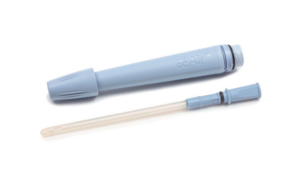
GentleCath Air for Women with FeelClean Technology protects against urethral damage and the risk of UTIs.

Extension to Indications for Use follows submission of results from a human factors study evaluating the safe and effective use of the device in the home care setting.