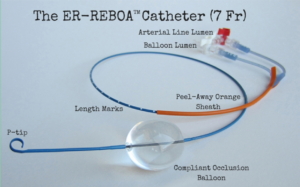
New FDA Cleared Balloon Catheter Used for Vessel Occlusion During Patient Resuscitation
Temporary vessel occlusion a growing practice for trauma patients.

Temporary vessel occlusion a growing practice for trauma patients.
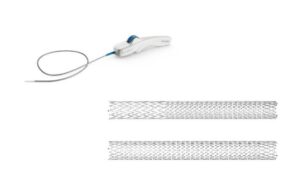
Philips (NYSE: PHG) today announced the first implant of its Duo venous stent system following FDA premarket approval.

Getinge announced today that it received FDA 510(k) clearance for its Talis +ACG (advanced clinical guidance) support software.
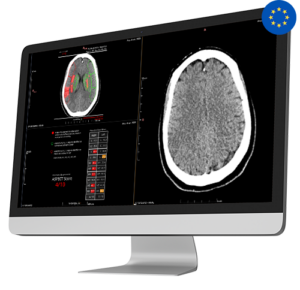
Medical imaging AI company receives approval for algorithm enabling opportunistic detection and triage of vertebral compression fractures.

Challenge Works today announces that Sysmex Astrego’s PA-100 AST System has won the $10m (£8m) Longitude Prize on AMR.
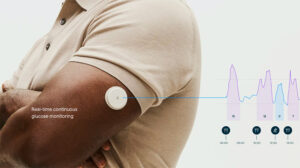
Abbott (NYSE: ABT)+ today announced FDA clearance for two over-the-counter continuous glucose monitor (CGM) systems, Lingo and Libre Rio.
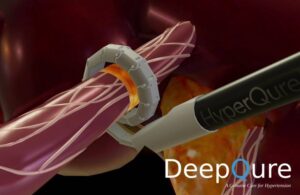
DeepQure announced today that it received FDA investigational device exemption (IDE) approval for a study of its HyperQure system.

CroíValve today announced favorable patient outcomes in a first-in-human clinical trial of its Duo tricuspid coaptation valve system.

Johnson & Johnson MedTech‘s DePuy Synthes announced today that it received a new FDA 510(k) clearance for its Velys surgical robot platform.

Mequ announced today that the FDA granted 510(k) clearance to its M Warmer system, a portable blood and IV fluid warmer platform.