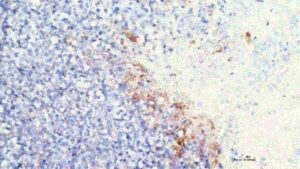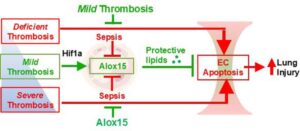
New potential gene-based treatment for sepsis and lung injury
A new discovery from the lab of YouYang Zhao, Ph.D., from Stanley Manne Children’s Research Institute at Ann & Robert H. Lurie Children’s Hospital of Chicago, opens promising new directions for treatment of life-threatening lung injury caused by sepsis.