
Edge Medical wins CE mark for surgical robot platform
Edge Medical announced on LinkedIn today that it received CE mark for MSP2000, its robotic-assisted “super system.”

Edge Medical announced on LinkedIn today that it received CE mark for MSP2000, its robotic-assisted “super system.”

FRANKLIN LAKES, N.J., Oct. 22, 2025 /PRNewswire/ — BD (Becton, Dickinson and Company) (NYSE: BDX), a leading global medical technology company, today announced a new self-collection solution for HPV testing in markets outside the United States. This new innovation simplifies at-home sample collection for patients and further automates lab processing using high-tech robotics with the BD COR™ System.
Researchers at the Icahn School of Medicine at Mount Sinai and their collaborators have developed a new technology to track beneficial bacteria after fecal microbiota transplants (FMT). The approach provides a detailed view of how donor microbes take hold and persist in the patients’ gut—not only which bacteria successfully colonized but how they change over time.

Today’s GPS smartwatches and other wearable devices give millions of runners reams of data about their pace, location, heart rate and more. But one thing your Garmin can’t measure is plain old physics: How much force is being generated when your foot hits the ground and takes off again.
A genomic test co-developed by Mayo Clinic and SkylineDx can identify whether people with melanoma are at low or high risk for cancer in their lymph nodes—a finding that could guide treatment decisions and help many people avoid lymph node biopsy surgery. The study results are published in JAMA Surgery.
Researchers led by investigators at Mass General Brigham and Massachusetts Institute of Technology have validated an ingestible capsule in preclinical models for the diagnosis of acute mesenteric ischemia, a condition caused by blocked or reduced blood flow to the intestines. The research is published in Science Robotics.
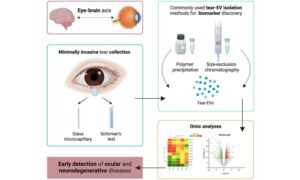
Ocular fluids provide a window into the health of the eye and the development of various pathologies. However, their study usually involves invasive techniques. “A wide range of eye diseases are being investigated, but the methods for obtaining aqueous humor and vitreous humor, the fluids inside the eye, are highly invasive, which limits their applicability in routine clinical practice,” explains Marta San Roque, Ph.D. student in the Innovation in Vesicles and Cells for Application in Therapy (IVECAT) Group at the Germans Trias i Pujol Research Institute (IGTP).
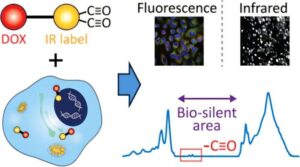
Scientists have made significant progress in developing cancer therapies that help patients across cancer types. However, they face limitations in determining the results of drug effectiveness, as well as ensuring even distribution among all cancer cells because of the highly compact nature of tumors. Researchers are working to change that by giving chemotherapy drugs a kind of chemical “signal” that allows them to be tracked inside of cells.
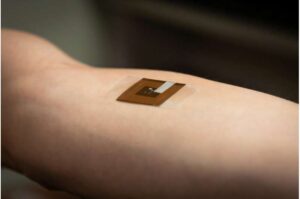
Researchers at Wake Forest University School of Medicine have developed a battery-free wearable patch that could help detect skin cancer earlier and more accurately, potentially saving lives by making screening more accessible and less invasive. The study was recently published in npj Biomedical Innovations.

ALLENDALE, N.J., Oct. 22, 2025 /PRNewswire/ — iNPLANT LLC, a leader in surgical innovation, today announced the release of the iNPLANT XL Funnel, a next-generation surgical device designed for the insertion of large breast implants (800cc–1500cc) using the “No-touch” surgical technique. The XL Funnel is the only device engineered specifically for this size range, addressing a critical unmet need in reconstructive and aesthetic breast surgery.