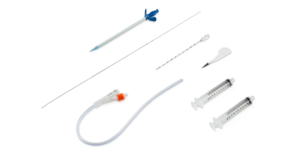
Mediplus expands S-Cath range with launch of S-Cath System Long
Mediplus has expanded its award-winning S-Cath System range for suprapubic catheterisation with the launch of S-Cath System Long, featuring longer components and enhanced features to give clinicians greater choice, flexibility, and improved usability.