
FDA clears Roche’s blood-based test for Alzheimer’s assessment
The blood-based biomarker test was developed in partnership with Eli Lilly and Company.

The blood-based biomarker test was developed in partnership with Eli Lilly and Company.

MSU’s Technology Transfer Office is facilitating the commercialisation process of the device.

Three presentations at ESMO 2025 will demonstrate Lunit SCOPE IO’s value in predicting immunotherapy response in colorectal, kidney, and lung cancer patients.
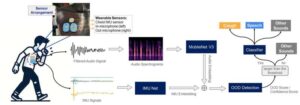
Researchers have improved the ability of wearable health devices to accurately detect when a patient is coughing, making it easier to monitor chronic health conditions and predict health risks such as asthma attacks. The advance is significant because cough-detection technologies have historically struggled to distinguish the sound of coughing from the sound of speech and nonverbal human noises.
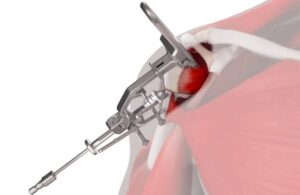
ohnson & Johnson MedTech (NYSE: JNJ)+
today announced the launch of its Inhance Intact shoulder arthroplasty instrumentation system.

Medtronic (NYSE: MDT)+
announced today that it received new labeling approval from the FDA for its Endurant stent graft system.
Health data helped identify kids in the ER who are likely to develop sepsis within 48 hours
OrthoIndy spine surgeon Dr Greg Poulter performed the inaugural SyncAR Spine case.
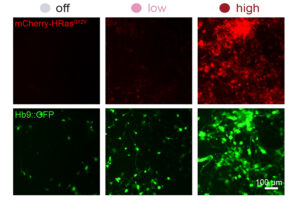
The promoter editing system could be used to fine-tune gene therapy or to more efficiently reprogram cells for therapeutic use.
SHANGHAI, Oct. 11, 2025 /PRNewswire/ — Smartee Denti-Technology today announced the global launch of the Smartee Sleep Aligners, a clear aligner device designed to help manage obstructive sleep apnoea hypopnoea syndrome (OSAHS) and primary snoring (PS). The new series includes two versions: Smartee SA, which focuses on the treatment of OSAHS and PS, and Smartee SA Plus. The latter can treat OSAHS while simultaneously addressing orthodontic issues. Together, they provide clinicians with an invisible alternative to conventional OSAHS treatment therapies, offering greater comfort and compliance, and improving sleep quality.