New injectable gel shows promise as voice loss treatment
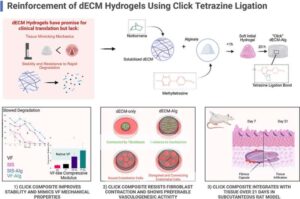
McGill University researchers have engineered a new hydrogel that shows early promise as a treatment for people with vocal cord injuries.

McGill University researchers have engineered a new hydrogel that shows early promise as a treatment for people with vocal cord injuries.

How do eggs get Salmonella? This bacteria poses a real threat to many eggs, but can easily be killed by cooking at a high heat.

Researchers at McMaster University have developed a new menstrual health product designed to complement and enhance an existing menstrual cup that is safer, easier to use and more environmentally sustainable than current options.
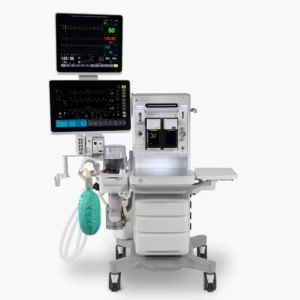
GE HealthCare (NYSE:GEHC) has unveiled Carestation 850, its next-generation anesthesia delivery system.
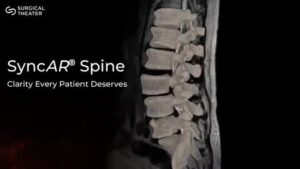
CLEVELAND, Oct. 10, 2025 /PRNewswire/ — Surgical Theater, the leader in surgical XR visualization, today announced U.S. Food and Drug Administration (FDA) 510(k) clearance for SyncAR® Spine, the next-generation release of its spine platform. This clearance expands the system’s capabilities with advanced XR tools powered by AI algorithms. This brings patient MRI and CT directly into the operating room, aligning preoperative plans with intraoperative execution for greater clarity and control in spine surgery.
Vitamin B6, which is absorbed from a broad range of foods, helps bolster immune system function and neurotransmitters in the brain. But some patients with chronic conditions like diabetes might experience low concentrations of vitamin B6, leading to reduced mental and physical health function, with possible symptoms including irritability, depression, anemia, numbness or muscle twitching.
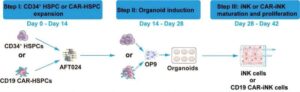
Chinese researchers have developed a novel method to efficiently engineer natural killer (NK) cells for cancer immunotherapy. NK cells are central to early antiviral and anticancer defense—among other immune system roles—making them well-suited for cancer immunotherapy.
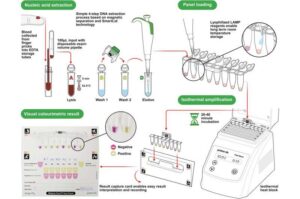
Researchers have adapted a rapid diagnostic technology that is able to identify undetected cases of malaria, helping tackle the spread of disease.

The xStep device offers painless, non-invasive electrical stimulation to the spinal cord to treat paralysis.
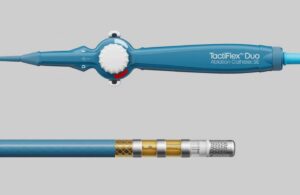
An Abbott (NYSE: ABT)+
official posted on social media to announce FDA breakthrough device designation for a new ablation catheter.