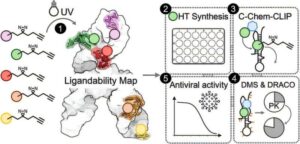
Antibody discovered that blocks almost all known HIV variants in neutralization assays
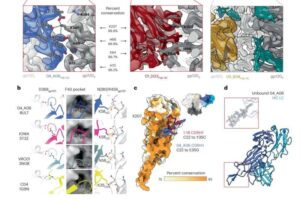
An international research team led by the University of Cologne has discovered an antibody that could advance the fight against HIV. The newly identified antibody 04_A06 proved to be particularly effective in laboratory tests. It was able to neutralize 98.5% of more than 300 different HIV strains, making it one of the broadest antibodies against HIV identified.