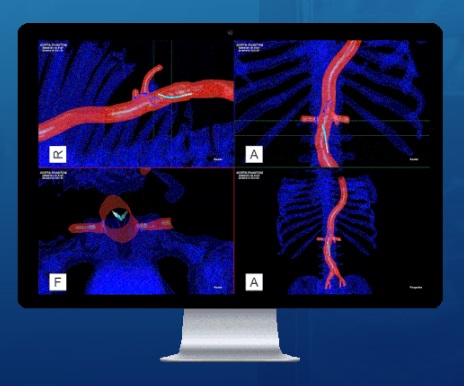
Viewpoint Catheter, a low profile 6 French (Fr) catheter available in multiple tip shapes and lengths, is designed for precision access to provide three-dimensional (3D) navigation feedback to clinicians. When Viewpoint Catheters are used in combination with the proprietary algorithms of the IOPS software, clinicians can clearly visualize endovascular tools in real-time and reduce their dependency on fluoroscopy systems. The result is state of the art image-guided, real-time navigation designed to revolutionize the way endovascular procedures are performed. This is achieved while reducing exposure to harmful radiation emitted from the x-ray fluoroscopy systems that are typically used to see vessels during these procedures.
At its core, the IOPS platform is designed to enhance visualization, minimize procedure times, and reduce total radiation exposure for the benefit of both patients and health care providers.