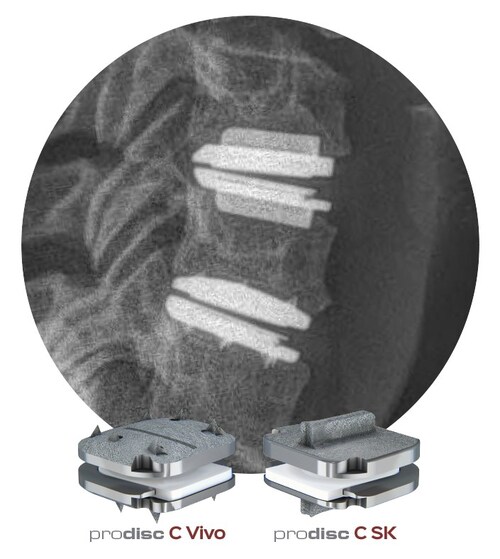
“It’s an exceptional day for cervical arthroplasty!” commented Armen Khachatryan MD, Orthopedic Spine Surgeon at The Disc Replacement Center in Salt Lake City, UT. “I am thrilled to witness the culmination of our efforts in the prodiscC Vivo and prodiscCSK clinical trial for two-level indications, resulting in FDA approval. I am particularly enthusiastic about integrating the distinct endplate configurations of the prodisc devices into my clinical practice. This approval expands the range of treatment options available to patients and underscores the continuous evolution and refinement of motion-preserving surgical techniques for cervical spine disorders,” Dr. Khachatryan concluded.
A total of 480 subjects were enrolled in the IDE Clinical Study across 31 centers, and the PMA is based on the analysis of 433 subjects—which is the highest number of subjects used to support a PMA for any cervical TDR device on the market. The Clinical Study was the first of its kind to evaluate two investigational TDR devices and the first PMA based on an IDE Clinical Study that compares an investigational system to a PMA approved cervical TDR device.