The trial will evaluate the safety and effectiveness of the eShunt System versus the current standard of care, the ventriculo-peritoneal (VP) shunt, in draining accumulated cerebrospinal fluid from the brain in elderly patients. The results of the STRIDE trial will serve as the basis for CereVasc’s anticipated submission to regulatory agencies for approval to market the eShunt System.
“A minimally invasive, endovascular approach to treating NPH has the potential to improve recovery times and reduce the possibility of post-operative complications for patients living with this progressive, neurological condition,” said Dr. Vitor Mendes Pereira, Neurosurgeon and Director of Endovascular Research and Innovation at St. Michael’s Hospital. “I am encouraged by initial study results of the eShunt System and look forward to participating in the STRIDE trial, with the goal of improving care and clinical outcomes for patients.”
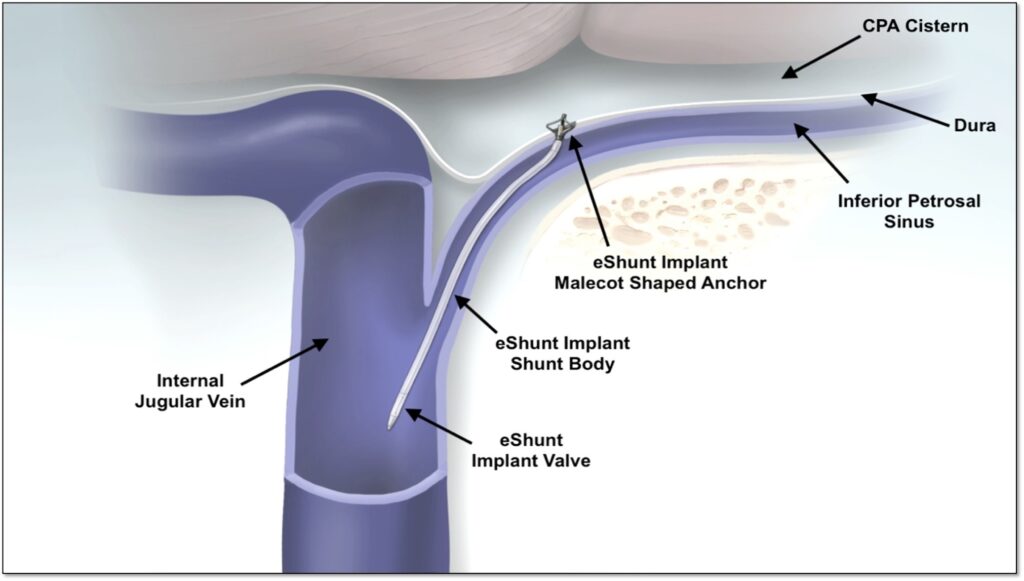
The eShunt System is the only minimally invasive, endovascular shunt and the first new treatment option developed for NPH since the VP shunt was introduced more than 60 years ago.