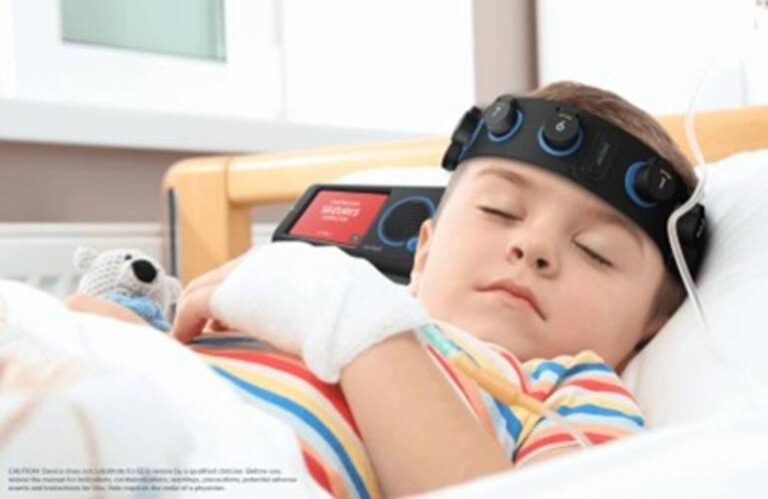
Clarity detects electrographic seizures in patients ages one and older. The company says this makes it the first and only AI-powered point-of-care electroencephalography (EEG) system cleared to detect electrographic seizures in patients at that age and to fully cover the age range from one year old to adult.
Using the technology, clinicians can detect non-convulsive seizures in pediatric patients in real time. This supports rapid diagnosis and treatment to help prevent serious brain injury.
CeriBell says Clarity now provides coverage across the largest age range ever addressed by a seizure detection technology. It offers hospitals a complete solution for detecting seizures, supported by a large-scale EEG database. Clarity works with the CeriBell EEG headbands, currently marketed for adults, but now available for patients of all ages.