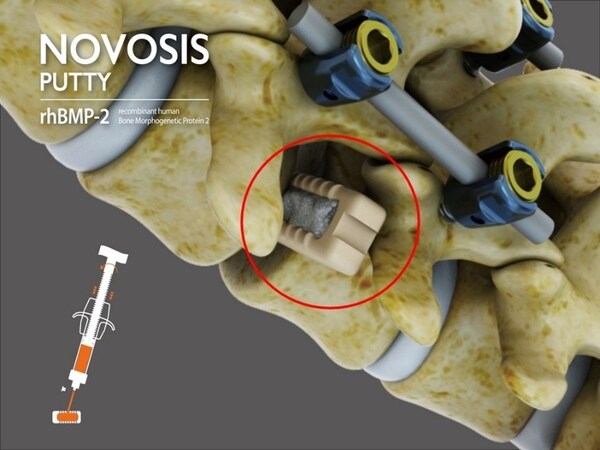
NOVOSIS PUTTY, previously designated as a Breakthrough Device by the FDA in December 2023, leverages novel technology to address unmet needs in bone regeneration. This milestone marks NOVOSIS PUTTY as the first Korean-developed bio-combined medical device to reach this stage in the U.S., signifying a significant step toward Premarket Approval (PMA) and subsequent commercialization.
The product features a dual-carrier system utilizing Hydroxyapatite (HA) and Tri-Calcium Phosphate (TCP), combined with CGBIO’s proprietary sustained-release technology, SLOREL™, to control the release of recombinant human bone morphogenetic protein-2 (rhBMP-2). This system is engineered to enhance high-density bone formation while minimizing ectopic bone growth, a common adverse effect in earlier rhBMP-2-based products. The safety and efficacy of NOVOSIS PUTTY have been validated in peer-reviewed publications, including the Journal of Clinical Medicine.