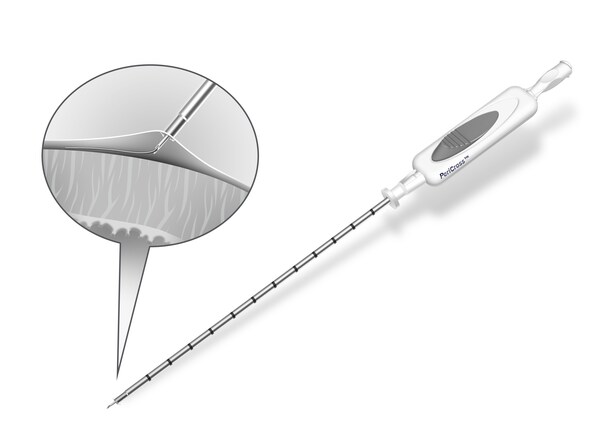
Epicardial access remains a technically demanding step in many electrophysiology and structural heart procedures. The PeriCross system features a unique tine-based retraction mechanism integrated with a 21-gauge micro-puncture needle, allowing physicians to create separation between the pericardium and myocardium prior to needle advancement—a design intended to improve control, support procedural consistency, and streamline workflow.
“The PeriCross system addresses one of the most challenging aspects of epicardial procedures with an elegant and intuitive design,” said Dr. Petr Neuzil, Director of the Cardiac Arrhythmia Service at Na Homolce Hospital in Prague. “Our early clinical experience has shown that the device performs reliably and efficiently across a range of patient anatomies.”