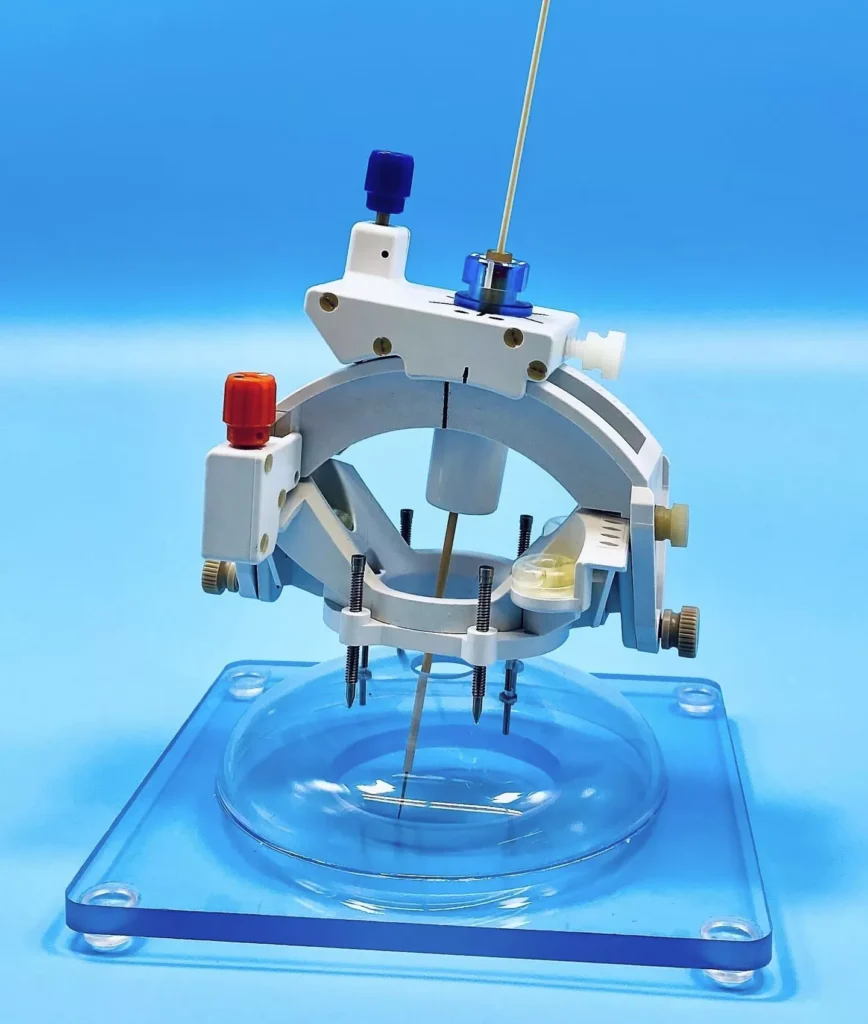
SOLANA BEACH, Calif., Jan. 16, 2024 (GLOBE NEWSWIRE) — ClearPoint Neuro, Inc. (Nasdaq: CLPT) (the “Company”), a global device, cell, and gene therapy-enabling company offering precise navigation to the brain and spine, today announced it has received 510(k) clearance for its SmartFrame OR™ Stereotactic System.
The SmartFrame OR Stereotactic System is composed of two main components: the SmartFrame OR, and the ClearPointer™ Optical Navigation Wand. The SmartFrame OR is intended to provide stereotactic guidance for the placement and operation of instruments or devices during planning and operation of neurological procedures performed in conjunction with the use of a compatible optical stereotaxic navigation system using preoperative MR and/or CT imaging. These procedures include biopsies, catheter placement and electrode introduction. The ClearPointer is intended to be used in conjunction with the SmartFrame OR and a compatible stereotactic optical navigation system for patient registration and navigation. SmartFrame OR may be used with or without available bone screw fiducials. The Company plans to commence limited market release in the first half of 2024, with a planned full market release in the second half of 2024.