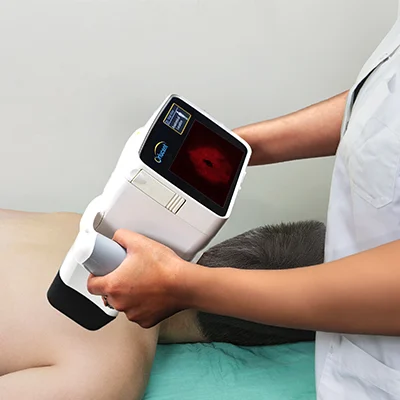
The results of the study validate the use of Orlucent’s SFI non-invasive, point-of-care molecular method to improve diagnostic accuracy by helping physicians classify difficult-to-characterize moles and carries great potential to reduce unnecessary biopsies and identify aggressive cancers early compared to current clinical practice.
“This is a major step forward for non-invasive skin cancer diagnostics,” said Douglas Grossman, MD, of Utah’s Huntsman Cancer Institute and the study’s lead author. “Skin Fluorescent Imaging lets doctors see melanoma molecular activity in the skin, which may help reduce unnecessary biopsies and improve early detection.”