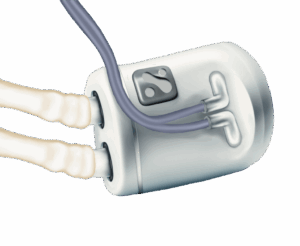
In an exclusive interview ahead of the official announcement, Nephrodite co-founder and CEO Nikhil Shah described the first-of-its-kind system as a “healthy alternative” to dialysis centers and at-home options.
The Atlanta-based device developer hopes to start its first-in-human study as soon as late 2027 and follow with a first regulatory approval as soon as early 2028, he said.
Shah — a former urologic oncology surgeon — founded the company in 2020 with Dr. Hiep “Bob” Nguyen, a pediatric urologist who serves as Nephrodite’s SVP of Science and Technology.