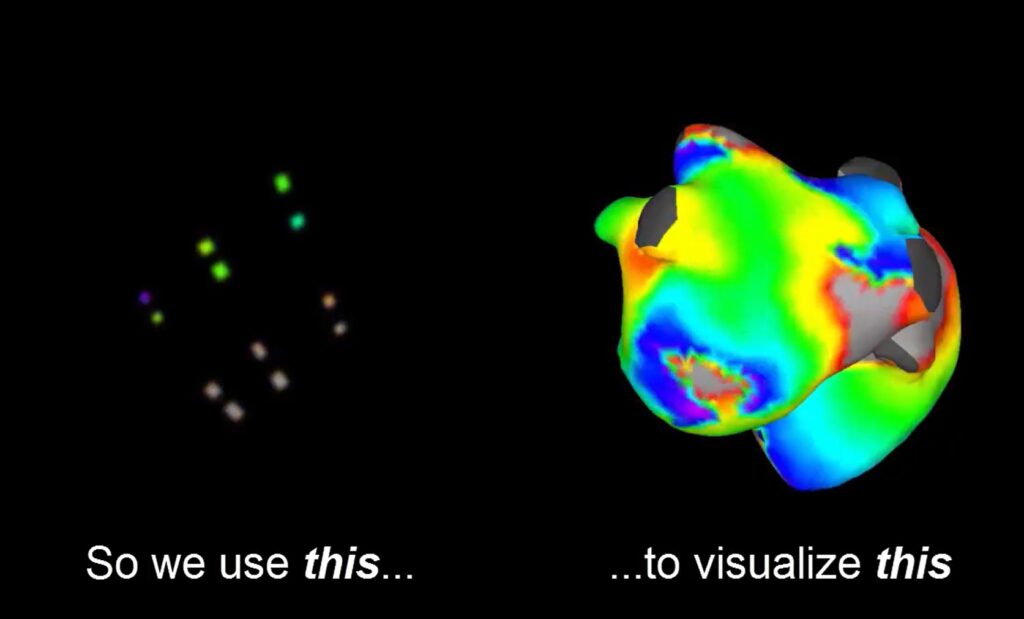
The study aims to evaluate the safety and effectiveness of the CoreMap endocardial EP mapping system in patients with AFib. It looks at both persistent AFib and long-standing persistent AFib. The company says that, to date, its mapping technology has demonstrated safety and acute effectiveness in more than 50 patients.
CoreMap designed its micro-electrode technology and novel algorithms to address current limitations in AFib mapping. Those include low spatial resolution and inadequate signal density, according to a news release.
The next-generation system guides patient-specific AFib ablation using ultra-high resolution endocardial mapping. Its Envenio EP mapping catheter features a dense array of micro-scale electrodes to capture highly localized, high-fidelity electrograms with minimal fractionation. This enables the use of novel algorithms to identify AFib drivers and support precise, targeted ablation.