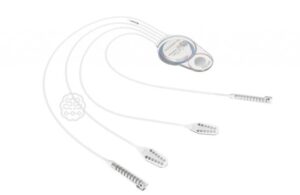
CorTec announced today that the FDA approved an investigational device exemption (IDE) application for its closed-loop brain-computer interface (BCI).
The IDE allows the University of Washington School of Medicine (UW) to evaluate CorTec’s Brain Interchange implant system in a clinical study. It will investigate the novel stroke rehabilitation treatment that uses cortical stimulation to enhance plasticity within the brain.
CorTec designed its Brain Interchange system to deliver fully implantable, closed-loop BCI to clinicians to investigate therapies. CTO Dr. Martin Schuettler believes the closed-loop functionality enables new possibilities for individualized treatments, according to a news release.