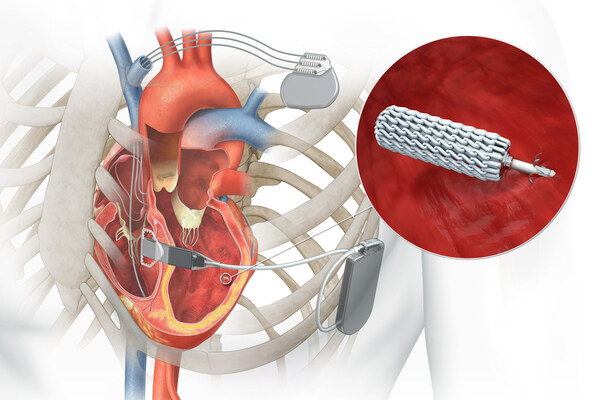
The FDA approved its WiSE cardiac resynchronization therapy (CRT) system through the premarket process. Australia-based EBR Systems — which has its U.S. base in Sunnyvale, California — says WiSE is the world’s only wireless cardiac pacing device for heart failure.
EBR designed its WiSE system to significantly expand the population of patients who could benefit from CRT. The leadless solution delivers left ventricular pacing, working seamlessly with existing pacemakers, defibrillators or CRT devices that provide right ventricular pacing.
The WiSE CRT system is the size of a cooked grain of rice.