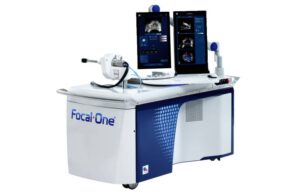
The new ultrasound imaging and workflow enhancements add to the company’s Focal One high-intensity focused ultrasound (HIFU) system. It further strengthen’s the Austin, Texas-based company’s leadership in robotic focal therapy for prostate cancer, according to a news release.
EDAP says the FDA clearance introduces advanced ultrasound imaging, streamlined treatment planning and an optimized user interface to the Focal One i system launched earlier this year. The company said its next-generation ultrasound imaging engine provides real-time visualization and supports the potential development of AI-driven algorithms to assist surgeons with tissue ablation visualization and treatment evaluation.