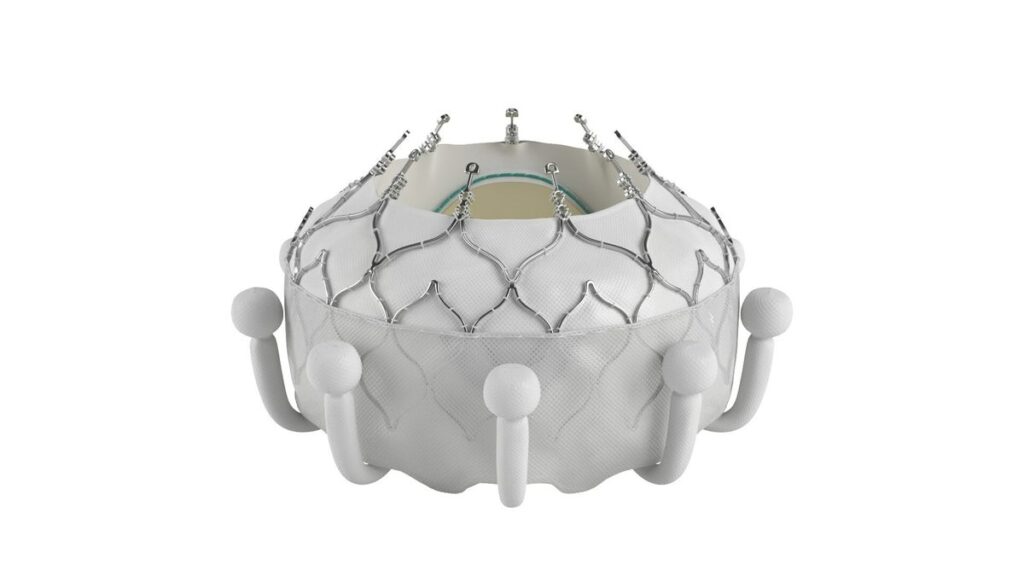
Edwards Lifesciences received approval from the Food and Drug Administration for its Evoque tricuspid valve replacement system, making it the first company to bring a transcatheter treatment for tricuspid regurgitation to the U.S.
Tricuspid regurgitation is a condition where the valve between the two right heart chambers doesn’t close properly, allowing blood to leak backward. Edwards’ Evoque device is inserted through a minimally invasive surgery and is designed to replace the tricuspid valve.
Competitor Abbott has submitted a device for FDA approval called TriClip, which clips together a portion of the leaflets of the tricuspid valve to prevent blood from leaking back. The FDA plans to hold an advisory panel on Feb. 13 to discuss the device.