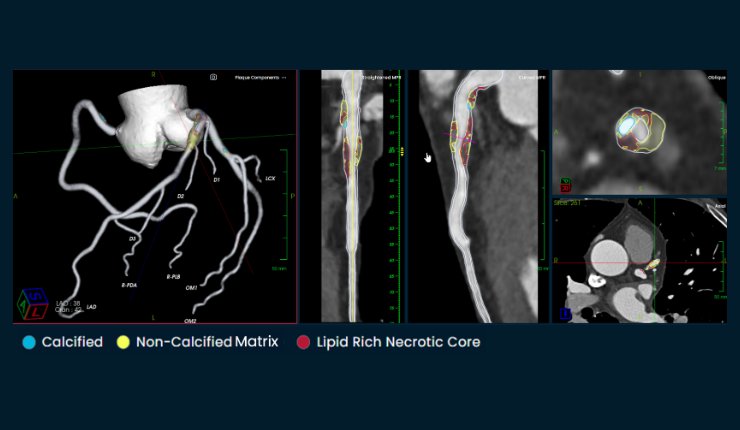
Elucid has announced it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its PlaqueIQ imaging analysis software to help physicians diagnose cardiovascular disease (CVD). Elucid says that PlaqueIQ is the first FDA-cleared non-invasive software that can objectively quantify and classify plaque morphology based on ground-truth histology, the gold standard for characterisation of plaques according to the company.
PlaqueIQ is designed to give physicians new, clinically validated information to help stratify patients and inform patient-specific treatment pathways.
Cardiovascular disease is the most common cause of death and disability globally, largely driven by myocardial infarction (MI) and ischemic stroke caused by atherosclerosis (plaque build-up and rupture in the arteries). While physicians look at many risk factors to evaluate patient risk, such as age, diet, and lifestyle, the strongest predictor of future events is the amount and type of plaque patients have in their arteries. Elucid says that around half of Americans between ages 45 and 84 have atherosclerosis and don’t know it.