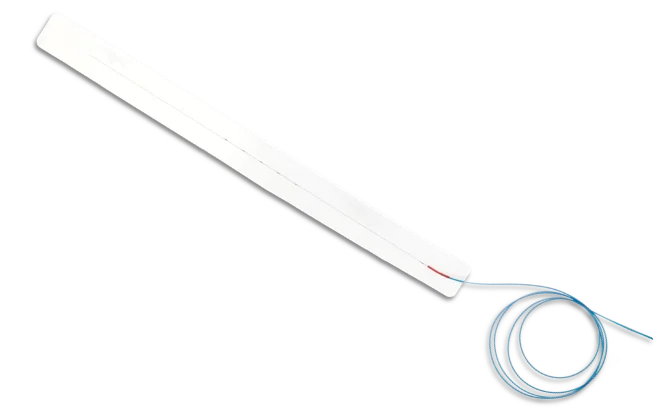
EmpNia has achieved a significant milestone with FDA clearance for its eMotus™ respiratory motion management system, designed to enhance precision and efficiency in image-guided radiation therapy (IGRT). Addressing one of radiation oncology’s most persistent challenges—accurately tracking respiratory motion—eMotus offers a novel solution using a disposable sensor pad paired with intuitive software. Unlike existing systems that are often costly, complex, and incompatible with various equipment setups, eMotus installs in hours, requires no capital-intensive infrastructure, and works seamlessly with all imaging and therapy modalities, making it accessible to a wider range of care settings.
The innovation lies in eMotus’ simplicity and universality. Its single-use disposable design eliminates cumbersome hardware and significantly reduces setup time, allowing clinicians to quickly implement respiratory motion tracking across all patient types. This not only improves workflow efficiency but also enhances treatment accuracy by providing reliable, real-time data. By eliminating compatibility issues and avoiding long installation periods, eMotus enables oncology teams to focus more on patient care and less on equipment management—directly addressing key pain points in radiation therapy delivery.