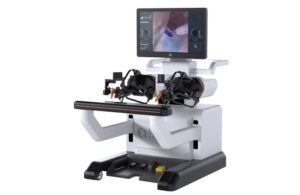
PARADIGM, a pivotal multicenter study, evaluates the company’s Endoluminal Surgical (ELS) system. It looks at ELS in lower gastrointestinal (GI) tract procedures by both colorectal surgeons and gastroenterologists.
The FDA granted investigational device exemption (IDE) for the study in December 2024. In May 2025, the company announced the first colorectal cases in the trial. In October 2025, the company reported the completion of the world’s first fully robotic endoscopic submucosal dissection (ESD) performed by a gastroenterologist in an IDE trial.
Now, the FDA says the company may proceed with the last stage of the pivotal multicenter trial. The decision follows the submission of a report on the safety results for the ELS system for the first set of subjects. Approval enables the completion of enrollment in the study ahead of a planned de novo classification request in the U.S.