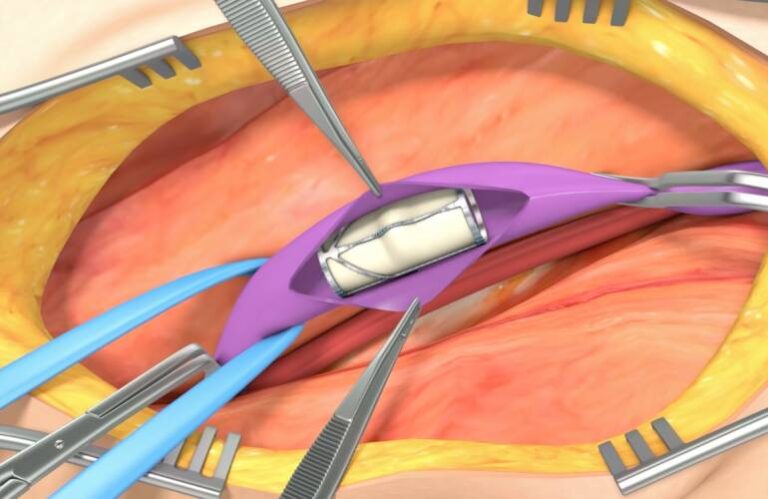
The company (formerly Hancock Jaffe) develops the VenoValve system. The potential first-in-class surgical replacement valve could provide a treatment for patients with severe deep venous chronic venous insufficiency (CVI). According to a news release, the company submitted an FDA pre-market authorization (PMA) application for the valve. It expects a decision in the second half of this year.
EnVVeno had its manuscript detailing three-year outcomes for its novel bioprosthetic valve published in the peer-reviewed Annals of Vascular Surgery journal. Findings covered the use of VenoValve heart valve implant in the treatment of deep venous reflux.