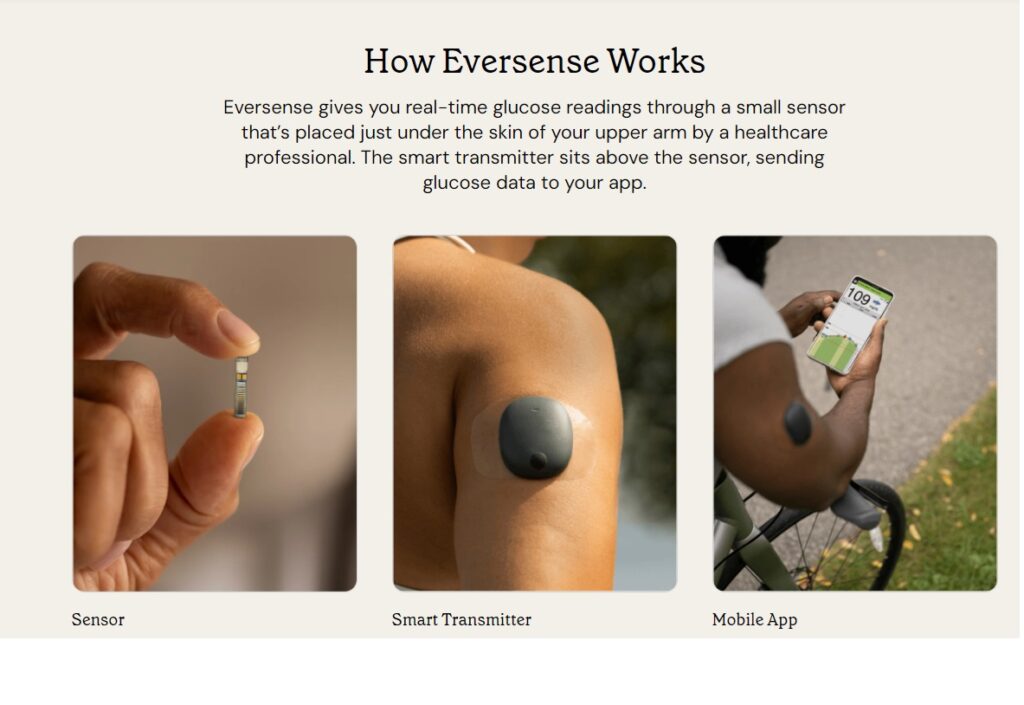
GERMANTOWN, Md. & PARSIPPANY, N.J.–(BUSINESS WIRE)– Senseonics Holdings, Inc. (NYSE American: SENS), a medical technology company focused on the development and manufacturing of long-term, implantable continuous glucose monitoring (CGM) systems for people with diabetes and Ascensia Diabetes Care, a global diabetes care company, announced that Eversense® has been granted an integrated CGM (iCGM) designation by the US Food and Drug Administration (FDA).
As the first fully implantable device in the category, Eversense has been authorized to be marketed as an iCGM through the FDA’s De Novo pathway, by establishing the special controls that will serve as a predicate device for 510(k) submissions in the future for devices of the same type.