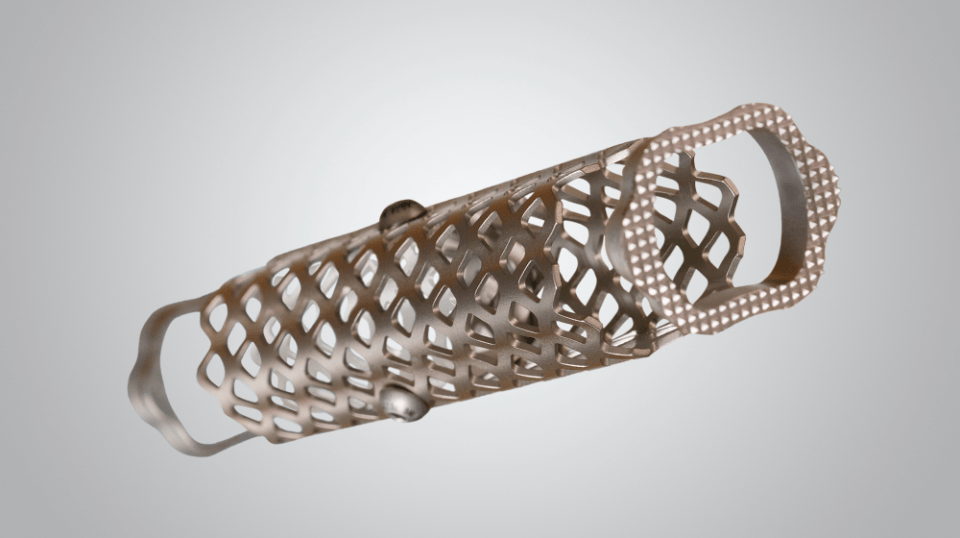
The modular, low-profile construct offers spine surgeons a flexible fixation solution for lateral and anterolateral lumbar fusions. The N-GAGE™ Plate uniquely pairs with the X-PAC® LLIF Expandable Interbody Cage System via a NON-SCREW-based, Polyaxial Coupler. This first-of-its-kind connection simplifies plate-to-cage attachment and allows anatomical conformity to the vertebral bodies, mitigating the limitations of rigid, screw-based-attached lumbar plates.
“This regulatory milestone not only reflects our ongoing commitment to solving persistent challenges that exist in spine surgery,” said Ron Sacher, Executive Chairman and Co-Founder. “It represents the first of multiple upcoming technologies that will transition EI from a product-based technology company to a complete procedural solutions provider. N-GAGE™ unlocks new revenue opportunities and end-user value to complement our flagship, NON-SCREW-based X-PAC® expandable technology platform.”