TAP provides early and frequent strategic engagement from the FDA, patients, providers and payers. It facilitates rapid development and widespread access to medical devices. Acceptance requires breakthrough device designation, which SetPoint Medical garnered last week.
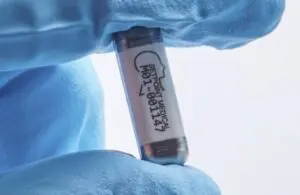
With acceptance into TAP, SetPoint can progress the development of its novel neuroimmune modulation platform. Valencia, California–based SetPoint designed its nerve modulation technology for people with relapsing-remitting multiple sclerosis (RRMS).
SetPoint designed its device to use precise vagus nerve stimulation. It activates anti-inflammatory and immune-restorative pathways to treat inflammation-mediated autoimmune conditions.