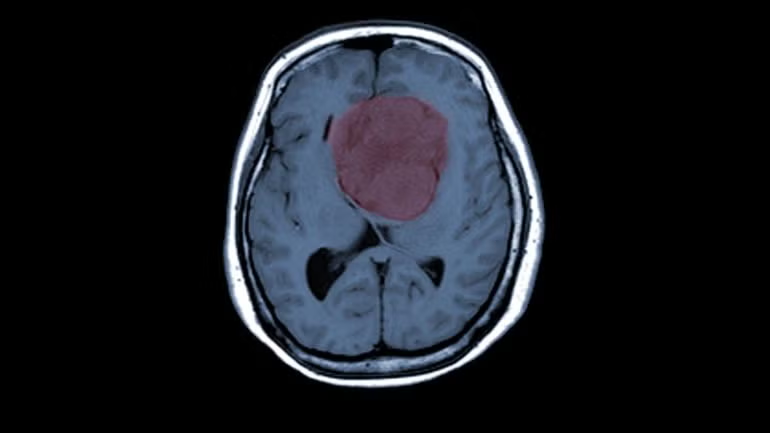
Zap Surgical’s ZAP Axon radiosurgery planning system has received 510(k) clearance from the US Food and Drug Administration (FDA), as well as gaining the European CE mark.
Achieved using radiosurgery systems such as Zap’s ZAP-X, Axon is specifically designed for planning cranial stereotactic radiosurgery (SRS) procedures. SRS involves directing radiosurgical beams from thousands of potential angles to precisely target and reduce the size of cancerous tumours while preserving healthy brain tissue.
According to Zap, Axon mitigates the need for clinicians to use multi-purpose planning software for SRS, despite it being originally developed for full-body applications. The company said that Axon delivers a more focused planning approach for clinicians with the aim of making radiosurgery case planning more efficient.