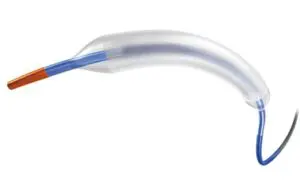
The DCB won approval for treating coronary in-stent restenosis (ISR) in patients with coronary artery disease. ISR occurs when plaque or scar tissue obstructs or narrows a stented vessel.
Marlborough, Massachusetts–based Boston Scientific plans a U.S. launch for Agent in the coming months. It already has availability in Europe, parts of Asia Pacific and Latin America. The system treats patients with ISR and previously untreated small vessel coronary disease.
Agent serves as an alternative to traditional therapies like balloon angioplasty, additional layers of stenting or radiation. The paclitaxel-coated balloon transfers a therapeutic dose of drug to the vessel wall, helping to prevent ISR reoccurrence.