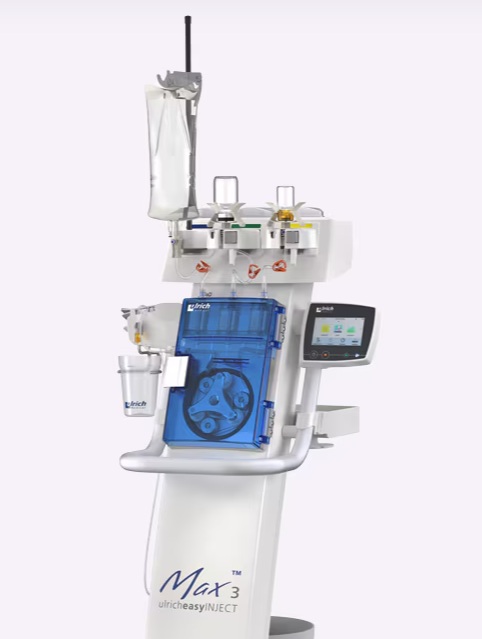
The Max 3™ injector is the first and only syringeless MRI injector available on the U.S. market. It is engineered for precise, efficient, and hygienic contrast delivery. The system simplifies MRI contrast administration with intuitive, step-by-step guidance via its user interface, helping technologists perform procedures quickly, confidently, and consistently. Its flexible design accommodates both single-use vials and multi-dose IBPs, allowing hospitals to tailor contrast delivery to their workflow, patient volume, and sustainability goals.
The FDA approval of Bracco’s VUEWAY® IBP provides a new option for hospitals seeking to improve workflow and reduce waste when using an FDA-Cleared automated contrast injection system, such as the Max 3™. With its multi-dose packaging design, VUEWAY® IBP enables the delivery of multiple single doses of contrast from one container, ensuring aseptic handling, reduced material waste, and more efficient use of contrast agents.