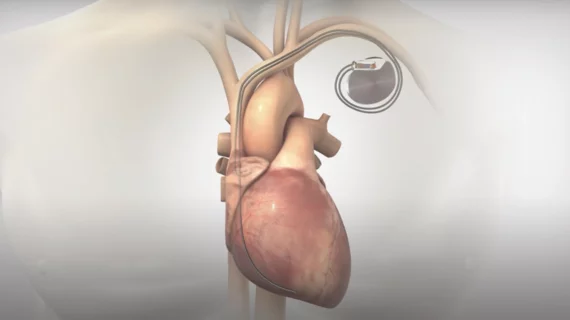
The FDA approved the protocol update, significantly expanding patient eligibility criteria for enrollment in the study. BACKBEAT evaluates the company’s atrioventricular interval modulation (AVIM) therapy. It looks at AVIM in pacemaker-indicated patients with uncontrolled hypertension despite the use of antihypertensive medications.
New Hope, Pennsylvania–based Orchestra won FDA breakthrough device designation for its AVIM therapy earlier this year. It uses conduction system pacing to immediately, substantially and persistently reduce blood pressure. The patented bioelectronic therapy comes through standard dual-chamber pacemaker.
Key updates include patients with Medtronic Astra or Azure dual-chamber pacemakers. This includes those devices with sufficient battery life implanted for any reason and those that are first device implants or implanted to replace an existing pacemaker. The new protocol also includes New York Heart Association (“NYHA”) class I or class II symptomatic heart failure.