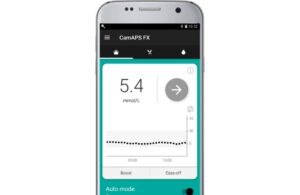
CamDiab announced today that the FDA granted authorization for its CamAPS FX advanced adaptive closed-loop artificial pancreas app.
CamAPS FX, an Android app, received approval as an interoperable automated glycemic controller device (iAGC). It helps to manage glucose levels in people with type 1 diabetes, aged two and older, including during pregnancy.
The app allows a compatible insulin pump and compatible continuous glucose monitor (CGM) to speak to one another, creating an artificial pancreas