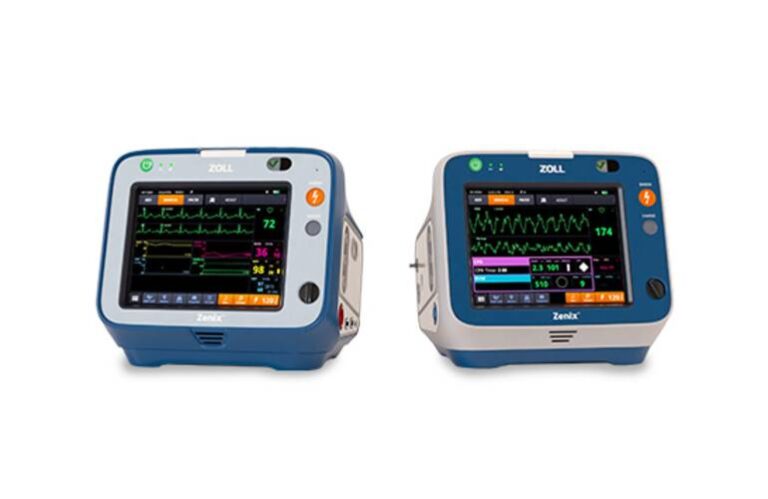
Chelmsford, Massachusetts-based Zoll, an Asahi Kasei company, said its Zenix system “redefines efficiency, clarity, and intelligence in both EMS and hospital settings.” The company built the device on years of feedback from customers, saying it combines intuitive design with powerful functionality.
According to Zoll, Zenix enhances patient care and automates workflows for ease of use. It features a large, durable touchscreen and provides critical information when needed. The system offers on-the-fly customization. It gives healthcare professionals the ability to make real-time adjustments, helping them to stay in control during high-pressure situations.
Zenix features Zoll’s Real BVM Help and Real CPR Help technology. These deliver real-time clinical feedback, improving ventilation quality and helping to provide high-quality CPR. The company said Zenix’s advanced technology empowers EMS teams and hospital clinicians to make informed, confident decisions. It helps to ensure they deliver proper patient care each time.