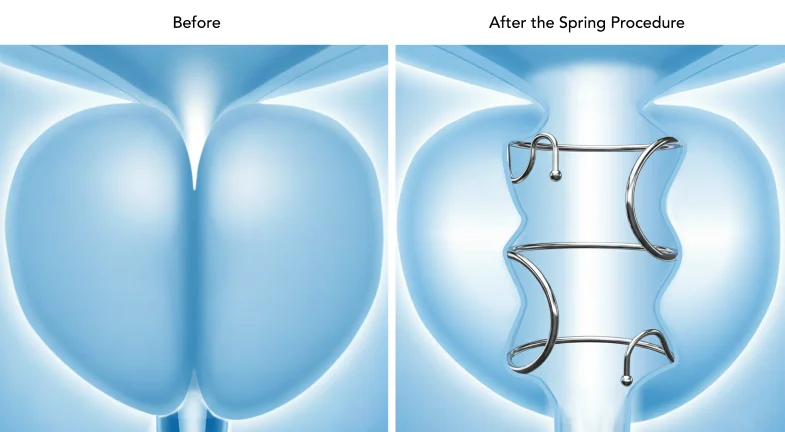
Zenflow, Inc. announced the U.S. Food and Drug Administration (FDA) approval of the Zenflow Spring Implant and Delivery System for the treatment of symptoms associated with benign prostatic hyperplasia (BPH), also known as enlarged prostate. The Zenflow Spring Implant and Delivery System is a novel, first-line interventional therapy (FIT) that provides urologists and their patients with a minimally invasive option that has proven long-term durability and unique safety advantages, including no post-procedure catheterisation and the ability to reverse treatment if desired.
Designed with the patient experience in mind, the Zenflow Spring Implant and Delivery System opens the urethra while preserving natural anatomy through a proprietary small spring-like implant. The Spring is the only FDA-approved FIT in a range of lengths and diameters, allowing for patient personalisation. It is made from a superelastic shape-memory material and delivered in an outpatient setting using the recently FDA-cleared Zenflow Spring Scope – a single-use, flexible cystoscope. This approval represents the very first FDA-approved BPH therapy delivered via a flexible cystoscope, which enhances patient comfort and recovery. Unlike other BPH interventions, the Zenflow Spring Implant and Delivery System eliminates burning, cutting, piercing, or resecting tissue. Zenflow’s unique design also offers complete reversibility without fear of complications commonly associated with other minimally invasive BPH treatments.