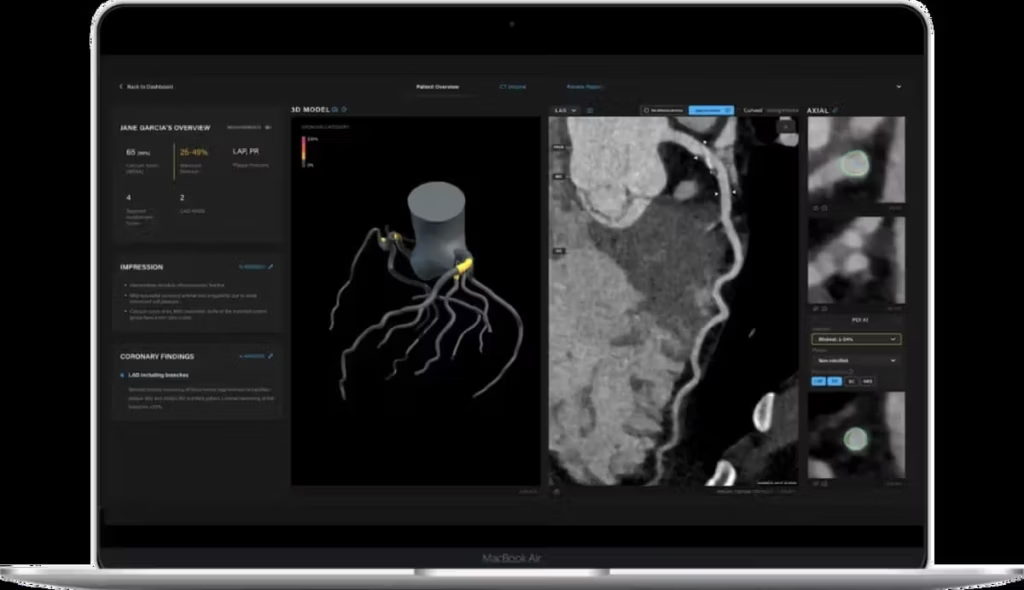
John Konstantopoulos, Co-Founder and CEO of Artrya, said:
“We are thrilled to have received FDA clearance of our Salix® Coronary Plaque module, which opens up a much greater revenue opportunity for us in the U.S., our largest market. The team have worked tremendously hard to prepare and support the submission which we lodged on the 16th of June, and we intend to build on this as we approach our next submission for the Salix® Coronary Flow module.
Our momentum is definitely building, with the core Salix® Coronary Anatomy platform now commercial in Tanner Health, and integration of Northeast Georgia Health System and Cone Health progressing well. Once live, we simply enable the Salix® Coronary Plaque module in their workstream, providing their clinicians access to our highly detailed assessment of coronary artery plaque in under ten minutes. We know these clinicians tremendously value the speed, ease and efficiency that our Salix® platform and plaque module offers, as they seek to rapidly diagnose patients and provide those in need with lifesaving treatment.