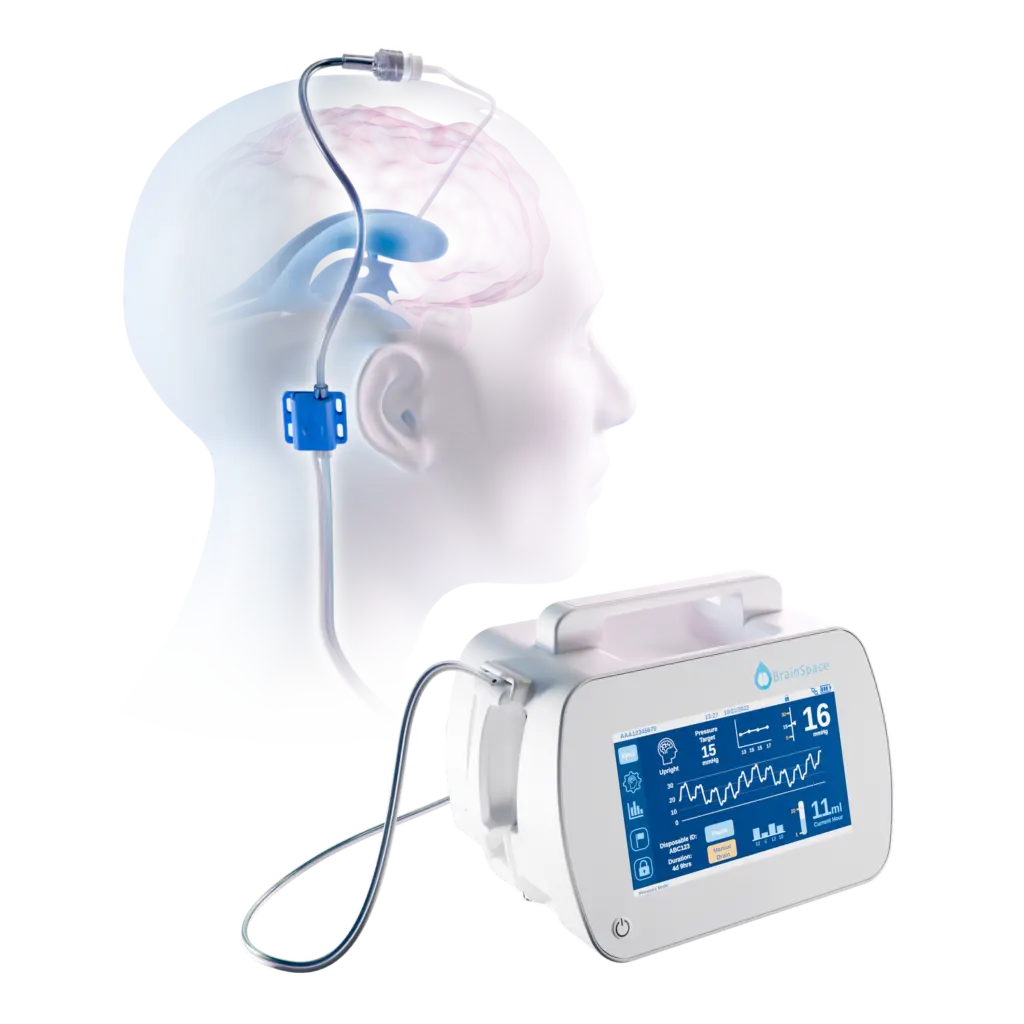
“We’ve heard from hundreds of ICU nurses that automating external CSF clearance is going to be a huge relief for nurses and a better experience for patients with Traumatic Brain Injury (TBI), Stroke, skull base tumor surgeries, neurodegenerative conditions like Normal Pressure Hydrocephalus and more,” shares Caitlin Morse, BrainSpace CEO & Co-founder. “That hope can now become a reality in hospitals around the US with Intellidrop FDA cleared.” The Intellidrop is indicated to provide external drainage of cerebrospinal fluid (CSF) and/or monitoring of CSF drainage and intracranial pressure (ICP) for ventricular or lumbar use.
According to a recent Lancet study, 1 in 3 people on the planet will face neuro injury, illness or degeneration in their lifetime. For these 2.5 billion people globally, better solutions can’t come soon enough. Time is Brain. The sooner a person can access appropriate care, the lower the risk that brain injury results in severe life-long disability. Automating brain pressure management simplifies the workflow for hospitals and staff. BrainSpace believes this will make care more accessible and allow for clinicians to introduce more personalized approaches.