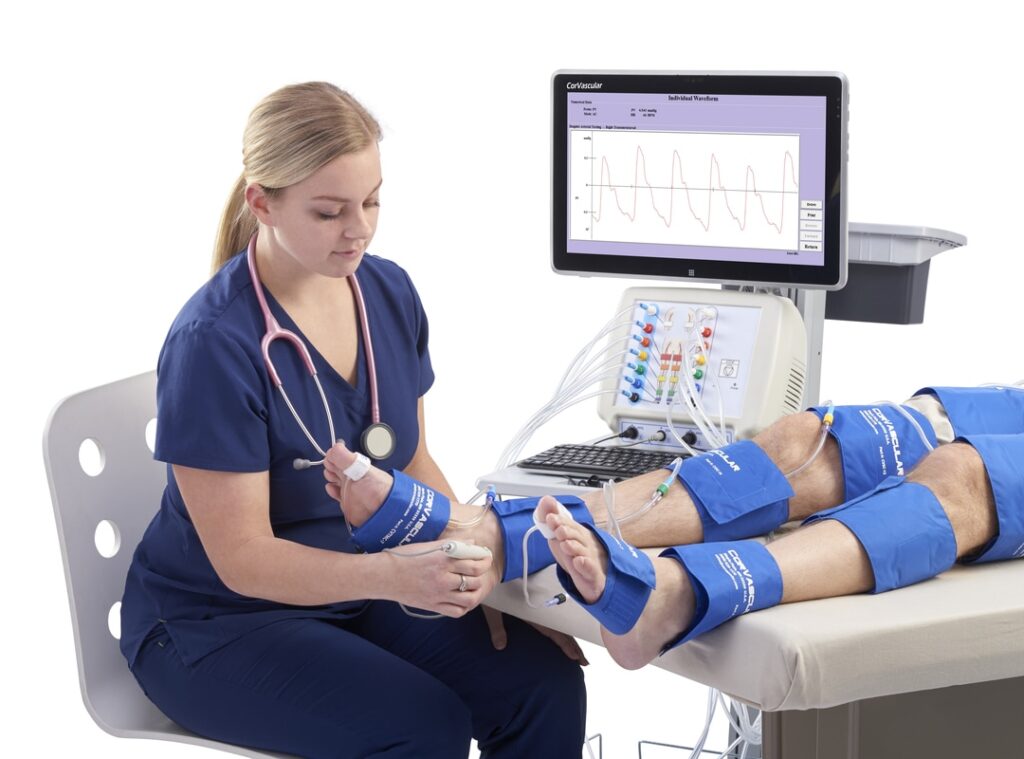
Minneapolis-based CorVascular plans to immediately begin marketing and selling the devices across the country. The newly cleared suite includes five devices, each with a unique combination of sensors and capabilities for physiologic testing. It features a device, touchscreen computer, mobile cart and a number of accessories approved as a system.
CorVascular developed the devices to test for peripheral arterial disease (PAD) and/or peripheral vascular disease (PVD). They could provide early identification to initiate guideline-directed medical therapy, potentially preventing cardiovascular disease, cerebrovascular disease and limb events such as amputation.