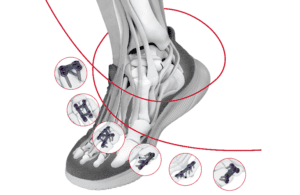
The company said it designed the TriLeap system to address the intricate needs of orthopedic surgeons and foot and ankle specialists.
As conditions like bunions become more prevalent —with Cleveland Clinic reporting that one in three U.S. residents are grappling with the disorder—elective foot surgeries are witnessing a surge. Plating systems like TriLeap play an important role in these surgeries, functioning as implants that stabilize the bones during procedures such as bunionectomies and osteotomies.
TriLeap offers a wide range of plate options tailored for various procedures and screw diameters, according to DePuy Synthes. Its instruments can be used during the reduction, internal fixation and fusion of bones and bone fragments.
“As a leader in elective foot and ankle procedures, DePuy Synthes is dedicated to developing novel solutions tailored to the changing needs of both patients and surgeons,” said Oray Boston, worldwide president of DePuy Synthes Trauma, Extremities, Craniomaxillofacial, Animal Health and Sports.