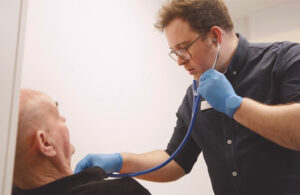
The medtech company specializes in advanced digital auscultation technology and has offices in Graz, Austria and Ottawa, Ontario. The technology assists healthcare providers using digital stethoscopes in screening, monitoring and diagnosing abnormalities in the heart, bowel and lungs in pediatric and adult patients through its machine learning-based, end-to-end encrypted web portal and mobile app.
In an interview ahead of tomorrow’s official announcement, eMurmur co-founder and CEO Dr. Andreas Schriefl said the auscultation software is designed to provide more objective analysis to a technique that relies on what providers hear, and assist physicians’ confidence in clinical decision-making, all in less time than traditional methods.