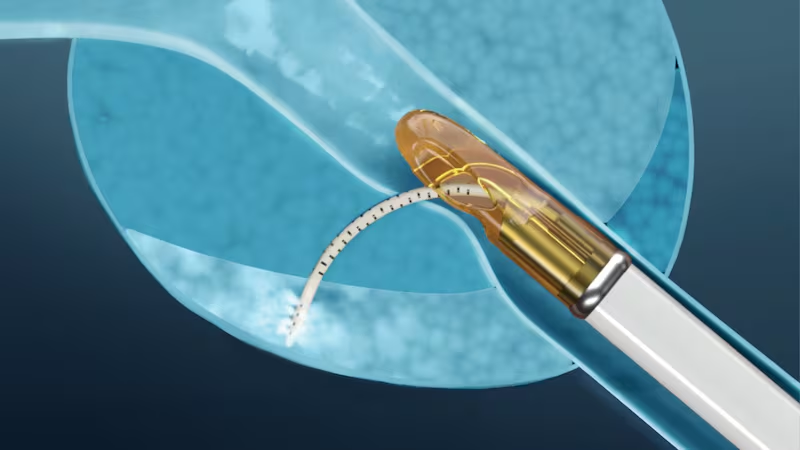
Francis Medical’s Vanquish device for treating intermediate-risk prostate cancer has been cleared by the US Food and Drug Administration (FDA).
Vanquish is a transurethral, ultrasound and electromagnetically guided thermal device that ablates cancerous prostate tissue using water vapour.
FDA clearance for Vanquish was based on data from US-based Francis Medical’s VAPOR 2 study (NCT05683691). The study evaluated the safety and efficacy of the Vanquish in treating subjects with Gleason Grade Group 2 (GGG2) localised intermediate-risk prostate cancer.