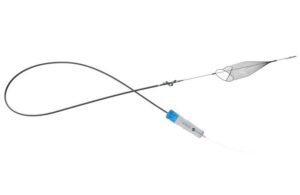
InterVene’s Recana, a mechanical thrombectomy catheter designed to address venous in-stent restenosis (ISR) and clear vessel obstructions, has received 510(k) clearance from the US Food and Drug Administration (FDA).
ISR, or the re-narrowing of a blood vessel, is most commonly seen in deep venous stent placement, particularly in patients with thrombotic pathology.