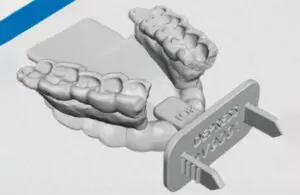
Stentra is fabricated of bio-compatible nylon materials (nylon ISO 10993-1), using 3D SLS printing technology.
Baltimore-based Kallisio says Stentra directs radiation to the target tumor area while reducing the harmful impact on surrounding tissue. It also immobilizes the tongue, lips, and other at-risk organs.
Stentra fabrication takes place in less than five days.