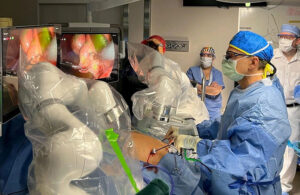
The latest MSS clearance is for laparoscopic cholecystectomies in pediatric patients ages 12 and older within a BMI range of 20-34 kg/m².
“For pediatric patients, minimizing surgical trauma is critical — less pain, faster recovery, and fewer scars all contribute to better long-term physical and emotional outcomes,” said Dr. Miguel Guelfand, professor and head of pediatric general and thoracic surgery at Cleveland Clinic Children’s.
Guelfand was part of the team that conducted that first pediatric cholecystectomy using MSS with Levita’s MARS (magnetic-assisted robotic surgery) system on Nov. 6.