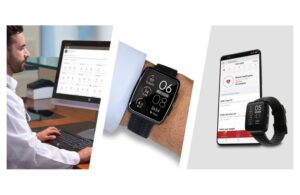
Clearance allows for the watch to integrate with the Masimo SafetyNet comprehensive telemonitoring system.
Last fall, the FDA cleared the W1 watch, which provides continuous oxygen saturation (SpO2) and pulse rate (PR) for over-the-counter and prescription use at home and in hospitals. This latest clearance enables accurate, reliable patient data, collected via the wrist-worn device, made available on the SafetyNet smartphone app. That enables review from remote caregivers anywhere and at any time.
W1’s capabilities come from the integrated Masimo MW-1 sensor, hardware, and software module. The watch features an integrated optical sensor and electrocardiogram (ECG) electrode pads that detect physiological signals. Masimo’s MW-1 module processes the signals using the company’s proprietary signal processing algorithms. This enables it to output high-resolution SpO2, pulse rate, perfusion index (Pi), and heart rate (HR) from an ECG.