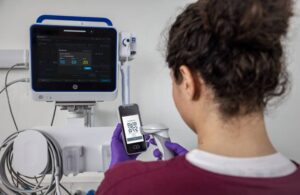
Portrait VSM provides clinicians with an accurate view of patient vital signs to support timely clinical decisions. It utilizes the SuperSTAT non-invasive blood pressure algorithm to provide precise and accurate measurement readings. Through wireless connectivity and seamless EMR integration, the monitor centers around workflow efficiency. It offers customized early warning scores (EWS) and enables care teams to focus on patient care by automating routine tasks.
The system builds on the GE HealthCare family of Portrait monitoring solutions. It can connect with the latest iteration of the Portrait Mobile wireless, wearable continuous monitoring system as well. Using a single click of a barcode scanner, Portrait VSM automatically imports the patient demographic data and a snapshot of the patient’s continuously monitored data, including respiratory rate, oxygen saturation and pulse rate, from Portrait Mobile.