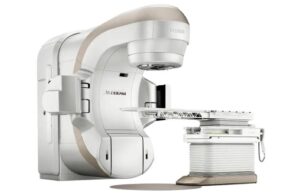
The Siemens Healthineers company designed HyperSight to empower clinicians to accurately tailor treatments to individual patients. This helps improve patient outcomes, thanks to new capabilities and workflows across the company’s linear accelerators.
HyperSight allows clinicians to acquire high-quality images during a patient’s daily course of radiation treatments. It helps to improve the ability to target tumor volumes more precisely and spare healthy tissue for patients undergoing radiation therapy.
Adding HyperSight to the Varian line of linear accelerators means the company’s portfolio can produce images that deliver the Hounsfield Unit (HU) accuracy necessary for treatment planning directly on the acquired conebeam CT (CBCT) images. This enables the use of the technology for offline adaptive planning to adjust for anatomical changes. These adjustments can take place over the course of treatment without the need for a trip to a separate CT scanner.