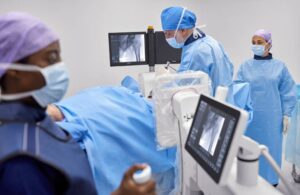
Zenition 30 reduces the dependency on support personnel by giving surgeons greater flexibility, control and personalization of C-arm movement and user settings. This can help to alleviate staff shortages that limit patient access and increase waiting times. It could also address budgetary constraints that affect today’s hospital systems.
With enhanced workflow efficiency through C-arm control alongside the patient table, surgeons can treat more patients. All the while, they can spend more time focusing on each patient, leading to a better patient and staff experience.
Zenition 30 features Philips’ latest-generation flat detector technology, advanced imaging algorithms and personalized user profiles. It delivers superior image quality, coupled with dose settings and a way of working from the moment users logo on. Fewer manual adjustments can lead to smoother procedures and more first-time-right imaging.